Abstract
Introduction: Over a three-year period, U.S. men with hemophilia were found to be 50 times more likely to die from renal disease than the general population (SMR 50; 95% CI 26.8-92.8) (Soucie et al., Blood 2000). Despite this finding, data regarding chronic kidney disease (CKD) and its risk factors in patients with hemophilia remain limited. The objective of this study is to determine the prevalence of CKD and CKD risk factors among older men with moderate and severe hemophilia.
Methods: This CKD cohort study is an extension of a U.S. national study sponsored by the American Thrombosis and Hemostasis Network (ATHN). The study, entitled ATHN 1: A Cross-Sectional Analysis of Cardiovascular Disease (CVD) in the Hemophilia Population, began enrollment in 10/2012. Inclusion criteria are men with moderate or severe congenital hemophilia A or B (FVIII or IX level ≤ 5%), age 54-73. Men with an additional bleeding disorder (besides liver dysfunction) were excluded. In this extension study, CKD risk factors, historical creatinine levels, and history of renal events were obtained from patient interview and chart review after obtaining informed consent. Glomerular filtration rate (GFR) values were calculated using the CKD-EPI equation and compared to values in the general population using the NHANES dataset (Coresh et al., JAMA 2007). CKD is defined as the presence of either kidney damage or decreased kidney function with GFR < 60 ml/min/1.73 m2 for ≥ 3 months, irrespective of cause.
Results: As of 6/30/2018, 134/200 planned subjects have been enrolled and interim analysis on 134 subjects from 18 U.S. hemophilia treatment centers (HTCs) is presented here. The majority were white (119; 88.8%) or African-American (13; 9.7%). Mean age was 64 years (SD: 5; range: 56-77). Most used factor on demand, with only 38.8% (52/134) on prophylaxis, defined as ≥2 doses of FVIII or ≥ 1 dose FIX/week. Four (3.0%) had a current inhibitor. Viral infection was common; 28.4% currently had hepatitis C, and 19.4% HIV. Hypertension (HTN) was reported in 51.5% of subjects, 14.9% diabetes mellitus (DM); and average BMI was 28.2 kg/m2 (36.6% obese). 11.6% (16/134) were found to have CVD (defined as angina, MI, TIA, or ischemic or embolic stroke).
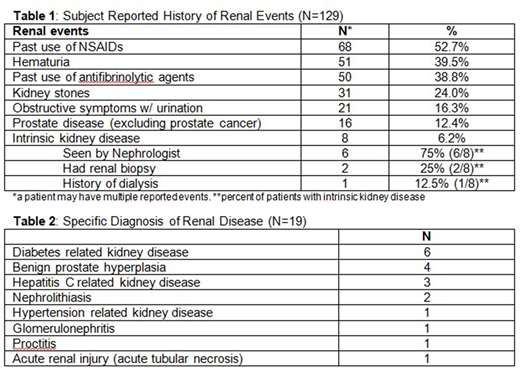
Acute kidney injury was common. Fasting blood work showed an abnormally elevated creatinine in 26.9% subjects (mean 1.1 mg/dl, SD 0.4). Mean historical maximum creatinine reported in the cohort was 1.0 (range 0.5-4.8), with mean GFR 67 (range 11-126). 11.4% (13/114) met the definition of CKD. Stages of CKD by GFR in the hemophilia cohort were similar to the NHANES general population (p=0.561). See Table 1 for subject reported history of renal events, and Table 2 for specific diagnoses of renal disease.
In our cohort, hemophilia subjects with CKD tended to have a diagnosis of intrinsic kidney disease (60.0% vs 11.6%, p=0.02), and non-significantly tended to have DM (23.1% vs 12.8%), age ≥65 years (21.1% vs 9.4%), and HTN (18.0% vs 9.8%) compared to subjects without CKD. No other significant trends were identified, including no association with CVD, HCV, HIV, BMI, or hematuria.
Conclusions: In this interim analysis of an ongoing national prospective cohort study, older men with moderate to severe hemophilia commonly report risk factors for CKD, including HTN (51.5%), DM, viral infection, and potential renal damaging medication use. Only 11.6% had CVD. Urological symptoms were also common, including hematuria and obstructive symptoms with urination.
In our cohort, 11.4% met the definition of CKD, defined as the presence of either kidney damage or GFR < 60 ml/min/1.73 m2 for ≥ 3 months. The distribution of GFR values appeared similar to the general population. As with risk factors associated with CKD in the general population, diagnosis of intrinsic kidney disease was significantly associated with CKD in hemophilia subjects, with non-significant trend for increased DM, older age, and HTN compared to subjects without CKD. It is reassuring that the prevalence of CKD does not appear to be increased in men with hemophilia compared to the general population, despite a known and unexplained high incidence of HTN in the hemophilia population. We plan to formally compare the prevalence of CKD and CKD risk factors with similarly aged men in the ARIC database once enrollment is complete, as understanding the risk factors that contribute to CKD is essential to halt its progression.
Sood:Bayer: Research Funding. Shapiro:BioMarin: Research Funding; Shire: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding; Prometic Life Sciences: Consultancy, Research Funding; Kedrion Biopharma: Consultancy, Research Funding; Sangamo Biosciences: Consultancy; Octapharma: Research Funding; OPKO: Research Funding; Daiichi Sankyo: Research Funding; Bayer Healthcare: Other: International Network of Pediatric Hemophilia; Bio Products Laboratory: Consultancy; Genetech: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bioverativ, a Sanofi Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kessler:Biomarin: Research Funding; Dimension Advisory boards: Membership on an entity's Board of Directors or advisory committees; DSMB: Membership on an entity's Board of Directors or advisory committees; Sangamo: Research Funding; Novo Nordisk: Honoraria, Research Funding; Octapharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Key:UniQure BV: Research Funding. Quon:Bioverativ, a Sanofi Company: Speakers Bureau; NovoNordisk: Consultancy, Speakers Bureau; Bayer: Consultancy; Shire: Speakers Bureau; Genetech: Consultancy, Speakers Bureau; Octapharma: Consultancy. Manco-Johnson:Novo Nordisk: Honoraria; CSL Behring: Honoraria; Bayer AG: Honoraria, Research Funding; Biogentek: Honoraria; Baxalta, now part of Shire: Honoraria. Cuker:Kedrion: Membership on an entity's Board of Directors or advisory committees; Genzyme: Consultancy; Spark Therapeutics: Research Funding; Synergy: Consultancy. Ragni:Novo Nordisk: Research Funding; Shire: Research Funding; MOGAM: Membership on an entity's Board of Directors or advisory committees; Sangamo: Research Funding; Alnylam: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sangamo: Research Funding; SPARK: Consultancy, Research Funding; CSL Behring: Research Funding; Biomarin: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bioverativ: Consultancy, Research Funding; SPARK: Consultancy, Research Funding. von Drygalski:UniQure BV, Bayer, Bioverativ/Sanofi, Pfizer, Novo Nordisk, Biomarin, Shire, CSL Behring: Consultancy. Kouides:UniQure: Other: DSMB; Octapharma: Research Funding. Escobar:Bayer, CSL Behring, Genentech, Hemabiologics, Kedrion, Novo Nordisk, Octapharma, Pfizer and Shire: Consultancy; Pfizer: Research Funding. Wang:Daiichi Sankyo: Consultancy, Other: Travel. Konkle:Shire: Research Funding; Genentech: Consultancy; Bioverativ: Research Funding; BioMarin: Consultancy; Pfizer: Research Funding; Gilead: Consultancy; Spark: Consultancy, Research Funding; CSL Behring: Consultancy; Sangamo: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.